Metallo-Phthalocyanines (MPc) form a family of planar organic molecules with a metal ion (or 2 hydrogen atoms) at its center. These molecules are thermally and chemically stable. It was found that metal phthalocyanines have diverse properties, which include optical properties (photoconductivity, dyes, photosensitizers in photodynamic cancer therapy), and electronic properties (gas, pressure, humidity sensors, photovoltaic applications, and electroluminescence devices)[1-4].
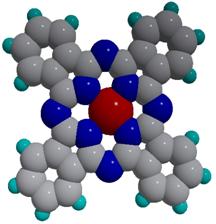
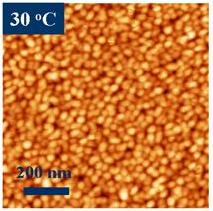
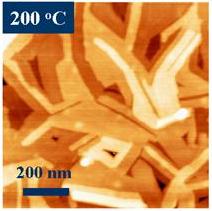 Figure 1. (a) Copper phthalocyanine molecule with 32 carbon (grey), 8 nitrogen (blue), 1 copper (red), and 16 hydrogen (cyan) atoms. The molecule is planar for light metals, such as Co, Cu, Ni, Fe, Mn. Atomic force microscopy images for iron phthalocyanine (FePc) deposited on a substrate kept (b) at room temperature (30 C) and (c) at 200 C. The grains are elongated and show molecularly flat plateaus in the case of the heated substrate. If deposited at room temperature, the grains are small and round.
Figure 1. (a) Copper phthalocyanine molecule with 32 carbon (grey), 8 nitrogen (blue), 1 copper (red), and 16 hydrogen (cyan) atoms. The molecule is planar for light metals, such as Co, Cu, Ni, Fe, Mn. Atomic force microscopy images for iron phthalocyanine (FePc) deposited on a substrate kept (b) at room temperature (30 C) and (c) at 200 C. The grains are elongated and show molecularly flat plateaus in the case of the heated substrate. If deposited at room temperature, the grains are small and round.While these classes of materials have been investigated for a long time, unprecedented control of their structure, electrical and magnetic properties have been recently accomplished using the Organic Molecular Beam Epitaxy (OMBE) available in our lab. The advance of thin film deposition of organics (OMBE) allows a control of the structural quantities, such as film thickness, grain size, and molecule orientation with respect to the substrate. For example, two parameters to control the structure of phthalocyanines are the substrate temperature during deposition and the type of substrate the phthalocyanine is sublimed onto (see Figure 1(b) and 1(c)). The resulting film morphology often impacts the electronic properties [5]. We are using high-resolution x-ray diffraction combined with quantitative refinement calculations to determine the precise orientation and stacking alignments of phthalocyanine molecules in thin films [6, 7].
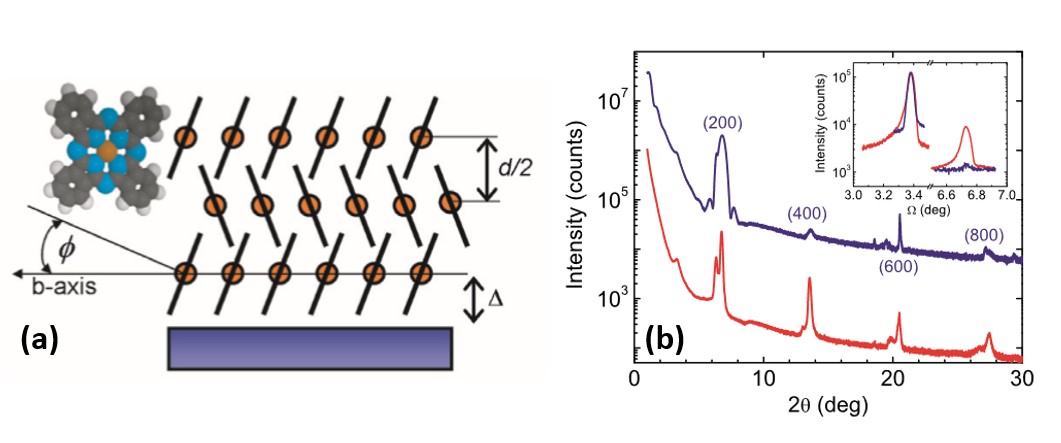 Figure 2. (a) The definitions of the tilt angle(φ), the substrate gap(∆) and the d-spacing of phthalocyanine (d) in a lattice of the α phase. For the β phase, the herringbone plane is parallel to the substrate surface. Circles represent the metal center and lines correspond to a side view of the nitrogen- and carbon-based molecule. (b) Experimental θ-2θ XRD profiles of OMBE-grown α-H2Pc (bottom) and α-CuPc (top) thin films on sapphire substrates. The CuPc is 36nm thick and was grown with the substrate temperature at 150◦C. The curve of CuPc is shifted vertically for clarity.
Figure 2. (a) The definitions of the tilt angle(φ), the substrate gap(∆) and the d-spacing of phthalocyanine (d) in a lattice of the α phase. For the β phase, the herringbone plane is parallel to the substrate surface. Circles represent the metal center and lines correspond to a side view of the nitrogen- and carbon-based molecule. (b) Experimental θ-2θ XRD profiles of OMBE-grown α-H2Pc (bottom) and α-CuPc (top) thin films on sapphire substrates. The CuPc is 36nm thick and was grown with the substrate temperature at 150◦C. The curve of CuPc is shifted vertically for clarity. The electronic properties of these organic semiconductors are explored for different geometric configurations, which include: sandwich (perpendicular current) [8], b) interdigitated electrodes (parallel current) [9], and c) organic field effect transistors (OFET). We find that chemical sensors (ChemFETs) for thin films have a superior chemical sensitivity than thicker films. The chemical response to various analytes (methanol, DMMP, H2O2, nitrobenzene, etc) is measured in a flow system with precise temperature and flow concentration control [10-13].
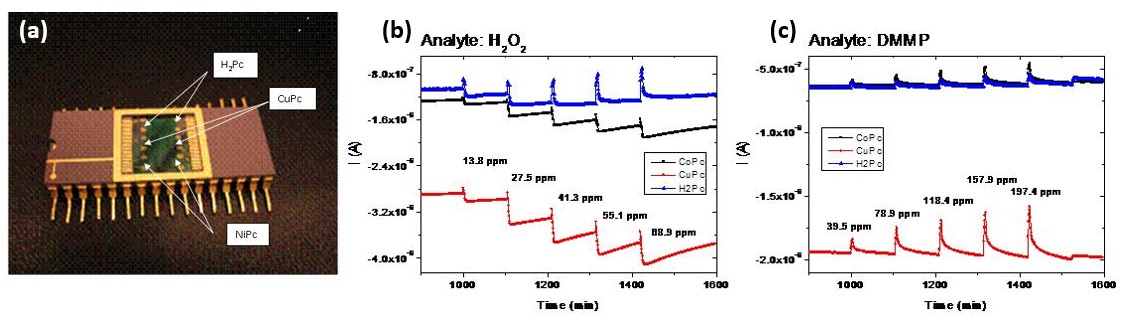 Figure 3. (a) Phthalocynine chemical sensor prepared at our lab consisting of 3 different types of metal organic layers (NiPc, CuPc and H2Pc). This design allows the detection and quantification (spectroscopy) of different chemical compounds. Sensor response at 50°C for different doses of (a) H2O2 and (b) DMMP. Sensor is exposed to the analyte for 300 seconds each time. The analyte concentration is depicted in the figure.
Figure 3. (a) Phthalocynine chemical sensor prepared at our lab consisting of 3 different types of metal organic layers (NiPc, CuPc and H2Pc). This design allows the detection and quantification (spectroscopy) of different chemical compounds. Sensor response at 50°C for different doses of (a) H2O2 and (b) DMMP. Sensor is exposed to the analyte for 300 seconds each time. The analyte concentration is depicted in the figure.Metallo-phthalocyanines (MPc), which incorporate many metal ions such as Fe, Cu, Co, Ni, H2 and Zn have been grown in our lab using OMBE and based on extensive studies we have been able to control their orientation and structure by appropriate choice of substrate and growth conditions [14, 15]. In contrast to commonly studied MPc powders, in thin films the structure and orientation of the M chains can be precisely tuned and controlled. This capability has opened up the possibility to study novel studies of low dimensional magnetism in MPc [5]. Magnetic characterizations, including MOKE, X-ray MCD and SQUID measurements, identified two temperature regimes, above and below ~4.5 K. The regimes are distinguished by short-range (paramagnetic) and long range (ferromagnetic) order due to intra and inter-iron-chain interactions.
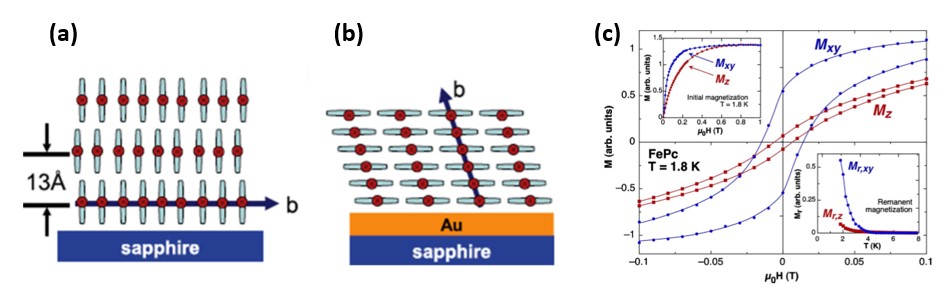
Figure 4. MPc thin film stacking on a supporting surface. (a) Standing configuration and (b) lying configuration. (c) Hysteresis loop of the FePc film deposited on Au covered sapphire measured at T=1.8 K in the direction perpendicular (Mz) and parallel (Mxy) to the substrate.
1. Lawton, E.A., The Thermal Stability of Copper Phthalocyanine. The Journal of Physical Chemistry, 1958. 62(3): p. 384-384.
2. Leznoff, C.C.a.L., A. P. B., Phthalocyanines – Properties and Applications, VCH, Editor. 1996: New York.
3. Allen, C.M., W.M. Sharman, and J.E.V. Lier, Current status of phthalocyanines in the photodynamic therapy of cancer. Journal of Porphyrins and Phthalocyanines, 2001. 5(2): p. 161-169.
4. Dimitrakopoulos, C.D. and D.J. Mascaro, Organic thin-film transistors: A review of recent advances. IBM Journal of Research and Development, 2001. 45(1): p. 11-27.
5. Bartolomé, J., et al., Highly unquenched orbital moment in textured Fe-phthalocyanine thin films. Physical Review B, 2010. 81(19): p. 195405.
6. Miller, C.W., et al., Quantitative structural analysis of organic thin films: An x-ray diffraction study. Physical Review B, 2005. 72(10): p. 104113.
7. Ge, L., T. Gredig, and I.K. Schuller, Origin of the anomalous X-ray diffraction in phthalocyanine films. EPL (Europhysics Letters), 2008. 83(5): p. 56001.
8. Colesniuc, C.N., et al., Exponential behavior of the Ohmic transport in organic films. Physical Review B, 2011. 83(8): p. 085414.
9. Miller, K.A., et al., Electrode Independent Chemoresistive Response for Cobalt Phthalocyanine in the Space Charge Limited Conductivity Regime. The Journal of Physical Chemistry B, 2006. 110(1): p. 361-366.
10. Yang, R.D., et al., Ultrathin organic transistors for chemical sensing. Applied Physics Letters, 2007. 90(26): p. 263506.
11. Bohrer, F.I., et al., Comparative Gas Sensing in Cobalt, Nickel, Copper, Zinc, and Metal-Free Phthalocyanine Chemiresistors. Journal of the American Chemical Society, 2009. 131(2): p. 478-485.
12. Park, J., et al., Ambient induced degradation and chemically activated recovery in copper phthalocyanine thin film transistors. Journal of Applied Physics, 2009. 106(3): p. 034505.
13. Yang, R.D., et al., Analyte chemisorption and sensing on n- and p-channel copper phthalocyanine thin-film transistors. The Journal of Chemical Physics, 2009. 130(16): p. 164703.
14. Serrano, A., et al., Effect of x-ray irradiation on Co-phthalocyanine thin films studied by surface plasmon resonance. Journal of Physics D: Applied Physics, 2016. 49(12): p. 125503.
15. Bartolomé, J., et al., Quadrupolar XMCD at the Fe $K$-edge in Fe phthalocyanine film on Au: Insight into the magnetic ground state. Physical Review B, 2015. 91(22): p. 220401.
